As they are non-biodegradable, plastics accumulate in the environment, altering habitats and natural processes. Millions of wildlife are also trapped by plastic waste every year.
When plastics break down, they release toxic compounds that contaminate the environment. They also disintegrate into small pieces of plastic called microplastics. Microplastics are now found all over the globe and are linked to severe health effects such as metabolic disorders and organ damage.
Recycling plastics reduces the amount of plastic waste that would otherwise be discarded and conserves natural resources. However, only about 10 per cent of plastic is currently recycled around the world. The figure is low in part because recycling some types of plastic, such as e-waste and marine plastic litter, is difficult. Chemical reactions that break down plastics into simpler components to be reused are also energy intensive.
From using e-waste plastics to culture cells to developing a greener method that breaks down plastics, researchers at NTU Singapore are solving some of the greatest challenges that stand in the way of recycling plastics. They are also making strides in reducing plastic pollution.
Repurposing e-waste plastics to grow “mini tumours” for laboratory testing
Plastics comprise a large portion of electronic waste (e-waste), and rapid technological advances, and high consumer demand drives its growing use in electronics. According to a UN report, the generation of e-waste is rising five times faster than the official recycling rate figures show. In 2022, e-waste generated 17 million tonnes of plastic globally.
Single-use plastics are also widely used in research and healthcare applications such as cell culture.
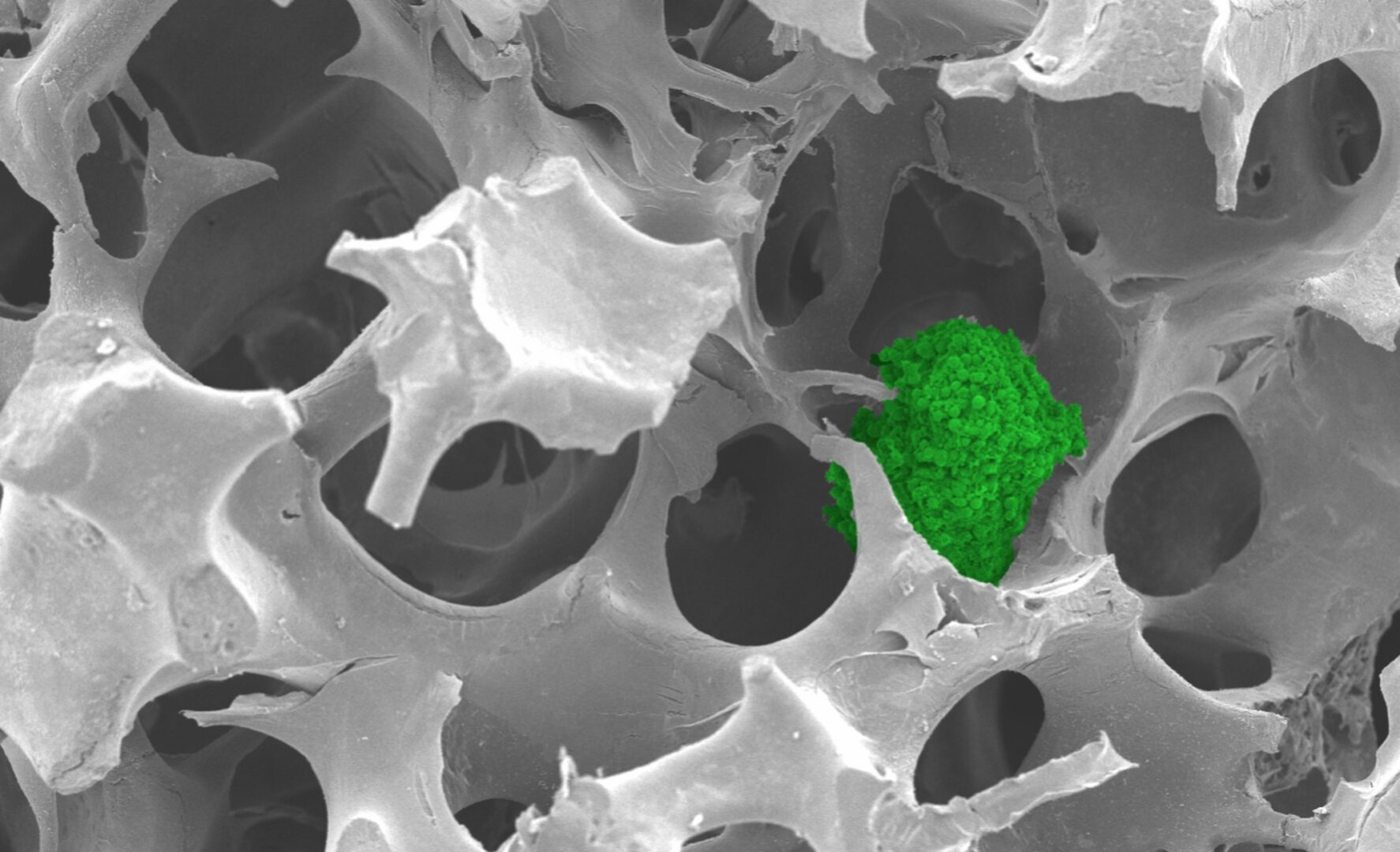
Cancer spheroid (green) growing in the matrix. Copyright: NTU Singapore.
Acrylonitrile butadiene styrene (ABS) is an e-plastic commonly used in the housings of devices such as keyboards and laptops. Repurposing plastics such as ABS for high value biomedical applications could be an attractive waste-to-resource strategy for effectively reducing plastic waste.
NTU scientists have developed a synthetic matrix to culture cells using ABS from discarded keyboards. The matrix is porous like a sponge and functions as a support structure, providing a framework for cells to attach and grow.
The matrix can culture spherical clusters of cells, called cancer spheroids, that resemble actual tumours. Due to their 3D shape, these “mini tumours” more accurately represent tumours than conventional cell cultures.
To fabricate the matrix, the scientists dissolved plastic scraps from discarded keyboards in an organic solvent, acetone and poured the solution into a mould.
The matrix supported the growth of breast, colorectal and bone cancer spheroids. The cancer spheroids had properties similar to those grown using commercially available matrices and may be used for biomedical applications such as drug testing.
“Our innovation not only offers a practical means to reuse e-waste plastics but could also reduce the use of new plastics in the biomedical industry,” said Assoc Prof Dalton Tay of NTU’s School of Materials Science and Engineering, who led the research.
The research was reported in Resources, Conservation & Recycling in 2024.
Converting hard-to-recycle plastic waste into hydrogen and carbon additives for polymer foams
While some types of plastics can be repurposed into new products, it is not as easy to recycle other kinds of plastics. Household plastics, packaging waste and marine plastic litter recovered from the environment are all examples of plastic waste that are difficult to recycle. There are also limited economic benefits to treating mixed and contaminated plastics.
Researchers from NTU explored using difficult-to-recycle plastics as a source of solid carbon material for application in polymer foams. The researchers first obtained gas and oil by heating different types of plastic waste at high temperatures (600 degrees Celsius) in the absence of oxygen. Then the gas and oil were heated at over 1000 degrees Celsius to break down the molecules into solid carbon and hydrogen. The solid carbon can be added to polymer foam to increase its strength and resistance to abrasion for cushioning applications. The foam containing the synthesised solid carbon derived from plastic waste exhibited properties comparable to other carbon-based and conventional reinforcing materials available on the market.
At the same time, the hydrogen produced could be collected and used as fuel.
Published in the Journal of Hazardous Materials in 2024, the research is a milestone in finding a use for plastic waste that previously could not be recycled. “We have developed a feasible approach to repurpose hard-to-recycle plastics, which is an important aspect of the circular economy,” said lead investigator Assoc Prof Grzegorz Lisak of NTU’s School of Civil and Environmental Engineering.
A bright way to break down plastics into valuable compounds
Although plastics can be broken down by heating them at high temperatures, such processes are energy intensive and generate greenhouse gases, contributing to global warming.

The experimental set-up where the dissolved plastic and vanadium catalyst solution is exposed to light, breaking down the plastic into useful compounds. Copyright: NTU Singapore.
Addressing the need for greener methods of breaking down plastics, NTU scientists have developed a process that can upcycle most plastics into chemical compounds useful for energy storage. The reaction uses light-emitting diodes (LEDs) and a commercially available catalyst and occurs at room temperature. It can break down a wide range of plastics, including polypropylene, polyethylene and polystyrene, all commonly used in packaging and discarded as plastic waste.
Compared to conventional plastic recycling methods, the process requires much less energy.
First, the plastics are dissolved in the organic solvent called dichloromethane, making the plastic polymer chains more accessible to the photocatalyst. The solution is then mixed with the catalyst and flowed through transparent tubes where LED light shines on it.
The light provides the initial energy to break the carbon-carbon bonds in a two-step process with the help of the vanadium catalyst. The plastics’ carbon-hydrogen bonds are oxidised, which makes them less stable and more reactive. After that, the carbon-carbon bonds are broken down.
The resulting end products are compounds such as formic acid and benzoic acid, which can be used to make other chemicals employed in fuel cells and liquid organic hydrogen carriers (LOHCs) – organic compounds that can absorb and release hydrogen through chemical reactions. LOHCs are being explored by the energy sector as a storage media for hydrogen.
According to Assoc Prof Han Soo Sen of NTU’s School of Chemistry, Chemical Engineering, and Biotechnology, who led the study, the breakthrough not only provides a potential answer to the growing plastic waste problem but also reuses the carbon trapped in these plastics instead of releasing it into the atmosphere as greenhouse gases through incineration.
The method was reported in the journal Chem in 2023.











